Chrome Plating
Chromium Electroplating or people just say
chrome plating is a process of metal coated using chromium substance or chromic acid. Why use chromium substance to cover metal surface? Chromium is a metal substance that resistant to corrosive while other metal like steel are easily to corrode affected by wet air. Chromium can’t be deposited from solution only chromic acid (CrO
3) and water. There must be present in bath one or more acid radicals which act catalyst to bring about or aid in the cathodic deposition of chromium. The purity of
chromic acid used is often not specified or established and yet the nature. The end of the process chrome will coated the surface of metal.
Chrome plating is not difficult covered on the part has been properly cleansed using the following requirements met:
- Preparation of the chromic acid (CrO3) solution..(Do not acquire the hydrogenated ( H3CrO4 ) chromic acid crystals)
- Temperature control of the bath (plating solution)
- Preparation of lead anodes (peroxide)
- Agitation method of the bath (bubbles)
- Plating current density control and duration (controller)
- Ventilation (for safety)
All that remains is the requirement of time - so don't let the apparent complexity of the task discourages you because the results are very worthwhile, indeed.
I have studied the industrial processes involved, reduced them to pint-size applications for model engineering, and experimented enough to be able to tell you what works. We have a lot to learn and the process has been laid out for you in ten easy steps. So, here we go!
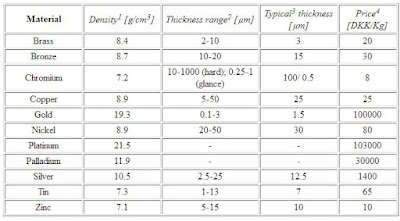
All chromium is about the same hardness; 800 to 1000 VHN - very hard! The main difference lies in the thickness of the deposit of chrome coated on the metal.
For decorative purposes, chrome sits best on nickel which itself adheres very well to copper - this combination also offers the best corrosion protection resistance more better. Decorative chrome coating thickness will vary from a few hundredths of a mil to 1 mil. The mirror finish will only be as good as the finish you put on the surface before you put on the chrome.
For functional purposes, to take advantage of the extremely low chrome coefficient of friction, or for wear build-up (bearing surfaces or pistons, as examples), hard chrome is plated in thickness as required from 1 to 50 mills.
When used as a bearing surface. Chrome must be micro-finished (more on this later) and will then provide a coefficient of friction lower than any other metal when used against steel, iron, brass, bronze, babbitt, or aluminium alloys. Do not use chrome against chrome. Because chrome is also much harder than casehardened steel, we then have a perfect set-up for longwearing working surfaces. Chrome will resist mostly all organic and in organic compounds and acids, except hydrochloric acid (muriatic).
Given fixed parameters for temperature, plating solutions, anodes, set-up, and current density, thickness is a function of time. Expect around .75 to 1.2 mil per hour of plating time.
I have plated up to 20 mills successfully at home - admittedly this was by accident because I was aiming for 3 mills deposit to refinish a piston! It had previously taken six hours using a particular chromic acid solution to deposit 3 mills of excellent chrome. I thought to shorten plating time I would increase the current density from 600 mA to 800 mA and the temperature of the solution was tweaked from 450
oC. to 500
oC. (113
oF to 122
oF). I then plated, with agitation, for five hours and wound up with an hour-glass shaped piston, due to a 13 mill chrome deposit measured at mid-skirt level and 21 mils on the edges (formed by the bottom of the skirt and the piston crown).
Let that be a lesson to all of us: Never change more than one parameter at a time. Subsequently, grinding of the same piston was successfully carried out; which attests to the excellent adhesion of the chrome to the base metal (steel) as prepared earlier.
Of course, the piston was then lapped to a perfect fit in the re-lapped bore (no rings involved in that 0.020 cu.in. engine). We'll come to the grinding and lapping notes later. Chrome will lap to a superb finish, to a degree of precision obtainable by no other method and limited only by the machinist's patience and skills.
NOTE: The chemical formulations given in this article are in avoirdupois ounces per gallon of solution (avoir. oz./gal). To convert these to metric measure, simply multiply the oz/gal number by the conversion factor of 7.5 to obtain grams per litre.
The formula for Bright Chrome Plating use the basic formulation of 100:1 chromic acid/sulphuric acid proportions:
- Chromic acid crystals = 33 oz. (936 grams)
- Sulphuric acid fluid = .33 oz. (9.36 millilitre)
- Distilled (or demineralized) water to make 1 gallon (3.79 litre).
Of course, you can vary these proportions in accordance with the quantity you wish to make up. So, to make up one pint for small work, simply divide everything by eight The dilution ratio of the sulphuric acid as purchased has to be taken into account and the amount used in the bath must be one of pure H
2SO
4 to 100 Cr0
3.
Be very accurate in this process; and:
ALWAYS ADD ACID SLOWLY TO WATER -- NEVER ADD WATER TO ACID
If you have access to demineralized water from your home dehumidifier (of course, clean filter if required). This is a good substitute for the recommended distilled water.
Also, I recommend the use of surgical rubber gloves when handling any of the chemicals called up in this article. Pharmacies (Chemists) carry them and they are much easier to replace than the skin of your hands.
The chromic acid crystals yield about 52% pure chrome metal. For reasons, which must remain unexplained at this stage, a freshly mixed solution will only deposit passably good chromium. The same solution, like a good wine, improves with age... So use it for experimentation when first mixed, before you undertake any serious plating - I keep mine in a sealed glass container and it is good for years. Filter as required between uses - plating current will be around 0.75 A/sq.in. For bright chrome and up to 1.4 A/sqin. for dull 'hard chrome'.
Black chrome plating can also be plated in the same way and still have similar characteristics to the bright chrome. For aesthetic or anti-reflective applications, it may be preferable in some cases. I have not yet used it, but the formula is as follows:
- Chromic acid 33 oz (936 g)
- Acetic acid = 28.2 oz (800 g)
- Barium acetate =1.0 oz (28 g)
- Distilled (or demineralized) water to make 1 gal. (3.79 litre).
- Operation of this bath will be at 90 ° to 115 °F (32.2 °C. to 46.1 °C.) and at a current density of 0.25 to 0.63 AIsq.in. (More on how to set this up later).
Temperature is critical for good (or any) results. This is best maintained automatically by using a thermostatically controlled electric heater right in the bath. A simple and cheap expedient for this requirement is to use a tropical fish-tank heater available at any pet store. And, while you're there, pick up a fish tank air pump, plastic piping to suit, and one air valve control, too.
The 115 V heater comes in a quartz tube with a temperature control knob on top. This acts on a bi-metal strip contact tension and can easily be cranked up to maintain the required 45 °C. to 50 °C. (113 °F. to 122 °F). A thermometer covering this range is also required.
It is important this temperature range be maintained throughout plating times.
Note: the above article I get from old literature but I forget to write the source. I also ever try to use the formula but not working smooth because use simple tools that I made by myself.
The product variety can be as on the picture below:
The process of chrome plating coloring is just the setting in variety of voltage and ampere of electric current and additive substance to make bright or smooth on coloring deposit.
 |
| Dull White Color Chrome Plating |
2. Bright White Chrome Plating
 |
| Bright Chrome Plating |
3. Gold Color Chrome Plating
 |
| Gold Color Chrome Plating |
 | | | | | | | |
| Gold Color Chrome Plating |
4. Dull Black Color Chrome Plating
 |
| Dull Black Chrome Plating |
5. Bright Black Chrome Plating
There are two different tipe of black chrome color from the chrome plating process, there are trivalen chrome plating process and hexavalent chrome plating process. Through trivalent chrome plating just can result semi black dark color, but this product have advantage of more stand to corrode. See
black and color chromium deposit.
Hexavalent chrome or Cr (IV) plating process can result true dark deposit color, very dark, smooth and non reflective. Hexavalent chrome is not good for goods that purpose to stand in corrosive environment because the layer is not strong enough stick to the surface layer.
 |
| Bright Black Chrome Plating |
Hard chrome applied on industry usually in their hydraulic shaft, this hydraulic need maintenance in certain period time need to coating again to repair the surface coating and adjust the hydraulic clearance.
 |
| Hard Chrome on Hydraulic Shaft |
Beside on hydraulic shaft, industrial uses also apply hard chrome on rolls use for certain uses like in transfer material in production process.
 |
| Hard Chrome in Rolls |
Hard chrome vanadium usually apply as coating on tools, this tool need hard surface in order not easily scratch when use to tight of open bolt from equipment.
 |
| Chrome Vanadium on Tools |